Vinegar Is An Aqueous Solution Of Acetic Acid . weak acids and bases may be just as dangerous as their stronger counterparts when concentrated. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. If the mass percent acetic acid in a bottle of vinegar is 9.0 % and the density of. For example, vinegar is an aqueous. vinegar is an aqueous solution of acetic acid. vinegar is an aqueous solution of acetic acid (ch3cooh). distilled white vinegar (figure \(\pageindex{2}\)) is a solution of acetic acid, \(ch_3co_2h\), in water. By law, it must contain 4 g acetic acid (ch3co2h 60.05g/mol) per 100 ml solution. acetic acid is a carboxylic acid with the formula ch3cooh, whereas vinegar is an aqueous solution of acetic acid and trace.
from www.numerade.com
By law, it must contain 4 g acetic acid (ch3co2h 60.05g/mol) per 100 ml solution. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. acetic acid is a carboxylic acid with the formula ch3cooh, whereas vinegar is an aqueous solution of acetic acid and trace. For example, vinegar is an aqueous. distilled white vinegar (figure \(\pageindex{2}\)) is a solution of acetic acid, \(ch_3co_2h\), in water. weak acids and bases may be just as dangerous as their stronger counterparts when concentrated. If the mass percent acetic acid in a bottle of vinegar is 9.0 % and the density of. vinegar is an aqueous solution of acetic acid. vinegar is an aqueous solution of acetic acid (ch3cooh).
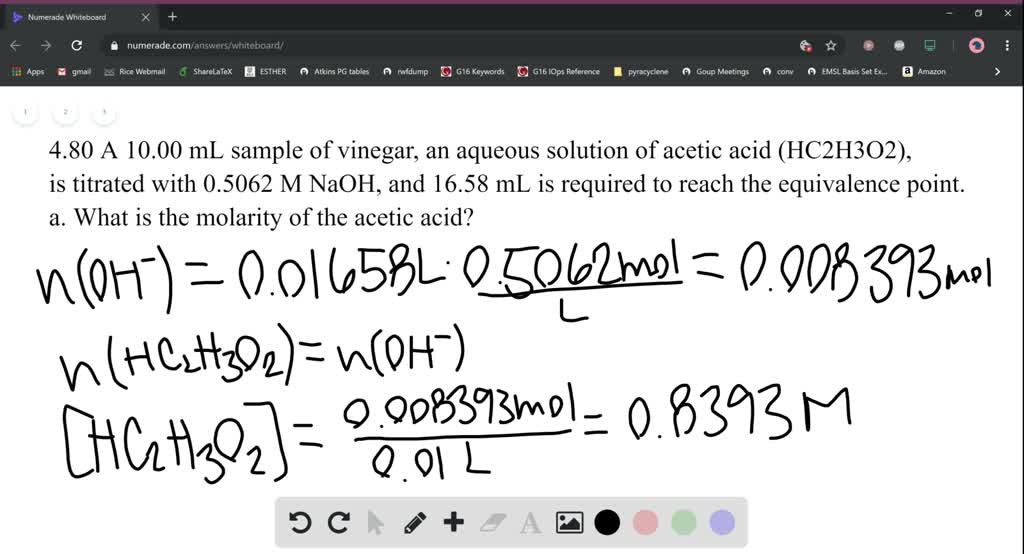
SOLVEDA 10.00\mathrm{mL} sample of vinegar, an aqueous solution of acetic acid \left(\mathrm
Vinegar Is An Aqueous Solution Of Acetic Acid By law, it must contain 4 g acetic acid (ch3co2h 60.05g/mol) per 100 ml solution. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. vinegar is an aqueous solution of acetic acid (ch3cooh). vinegar is an aqueous solution of acetic acid. weak acids and bases may be just as dangerous as their stronger counterparts when concentrated. distilled white vinegar (figure \(\pageindex{2}\)) is a solution of acetic acid, \(ch_3co_2h\), in water. For example, vinegar is an aqueous. acetic acid is a carboxylic acid with the formula ch3cooh, whereas vinegar is an aqueous solution of acetic acid and trace. If the mass percent acetic acid in a bottle of vinegar is 9.0 % and the density of. By law, it must contain 4 g acetic acid (ch3co2h 60.05g/mol) per 100 ml solution.
From www.dreamstime.com
Acetic Acid Molecule. Vinegar is an Aqueous Solution of Acetic Acid. Skeletal Formula. Stock Vinegar Is An Aqueous Solution Of Acetic Acid weak acids and bases may be just as dangerous as their stronger counterparts when concentrated. By law, it must contain 4 g acetic acid (ch3co2h 60.05g/mol) per 100 ml solution. If the mass percent acetic acid in a bottle of vinegar is 9.0 % and the density of. in this experiment, a technique known as a titration will. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.alamy.com
Acetic acid molecule. Vinegar is an aqueous solution of acetic acid Stock Photo 90598970 Alamy Vinegar Is An Aqueous Solution Of Acetic Acid weak acids and bases may be just as dangerous as their stronger counterparts when concentrated. vinegar is an aqueous solution of acetic acid. By law, it must contain 4 g acetic acid (ch3co2h 60.05g/mol) per 100 ml solution. distilled white vinegar (figure \(\pageindex{2}\)) is a solution of acetic acid, \(ch_3co_2h\), in water. For example, vinegar is an. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.dreamstime.com
Acetic Acid Molecule. Vinegar is an Aqueous Solution of Acetic Acid. Skeletal Formula Stock Vinegar Is An Aqueous Solution Of Acetic Acid vinegar is an aqueous solution of acetic acid. If the mass percent acetic acid in a bottle of vinegar is 9.0 % and the density of. By law, it must contain 4 g acetic acid (ch3co2h 60.05g/mol) per 100 ml solution. in this experiment, a technique known as a titration will be used to determine the concentration of. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.chegg.com
Solved 1) When an aqueous solution of acetic acid (vinegar) Vinegar Is An Aqueous Solution Of Acetic Acid in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. acetic acid is a carboxylic acid with the formula ch3cooh, whereas vinegar is an aqueous solution of acetic acid and trace. vinegar is an aqueous solution of acetic acid. If the mass percent acetic acid in. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.chegg.com
Solved 2) Household vinegar is an aqueous solution of acetic Vinegar Is An Aqueous Solution Of Acetic Acid vinegar is an aqueous solution of acetic acid. acetic acid is a carboxylic acid with the formula ch3cooh, whereas vinegar is an aqueous solution of acetic acid and trace. weak acids and bases may be just as dangerous as their stronger counterparts when concentrated. in this experiment, a technique known as a titration will be used. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.alamy.com
Acetic acid molecule. Vinegar is an aqueous solution of acetic acid. Skeletal formula Stock Vinegar Is An Aqueous Solution Of Acetic Acid acetic acid is a carboxylic acid with the formula ch3cooh, whereas vinegar is an aqueous solution of acetic acid and trace. weak acids and bases may be just as dangerous as their stronger counterparts when concentrated. By law, it must contain 4 g acetic acid (ch3co2h 60.05g/mol) per 100 ml solution. in this experiment, a technique known. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.alamy.com
Acetic acid molecule. Vinegar is an aqueous solution of acetic acid. White skeletal formula on Vinegar Is An Aqueous Solution Of Acetic Acid If the mass percent acetic acid in a bottle of vinegar is 9.0 % and the density of. For example, vinegar is an aqueous. vinegar is an aqueous solution of acetic acid. distilled white vinegar (figure \(\pageindex{2}\)) is a solution of acetic acid, \(ch_3co_2h\), in water. acetic acid is a carboxylic acid with the formula ch3cooh, whereas. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.dreamstime.com
Acetic Acid Molecule. Vinegar is an Aqueous Solution of Acetic Acid. Skeletal Formula Stock Vinegar Is An Aqueous Solution Of Acetic Acid vinegar is an aqueous solution of acetic acid (ch3cooh). in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. distilled white vinegar (figure \(\pageindex{2}\)) is a solution of acetic acid, \(ch_3co_2h\), in water. For example, vinegar is an aqueous. By law, it must contain 4 g. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.alamy.com
Acetic acid molecule. Vinegar is an aqueous solution of acetic acid. Stylized skeletal formula Vinegar Is An Aqueous Solution Of Acetic Acid vinegar is an aqueous solution of acetic acid (ch3cooh). weak acids and bases may be just as dangerous as their stronger counterparts when concentrated. vinegar is an aqueous solution of acetic acid. distilled white vinegar (figure \(\pageindex{2}\)) is a solution of acetic acid, \(ch_3co_2h\), in water. For example, vinegar is an aqueous. By law, it must. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.numerade.com
SOLVED A typical sample of vinegar has a pH of 3.0 . Assuming that vinegar is only an aqueous Vinegar Is An Aqueous Solution Of Acetic Acid vinegar is an aqueous solution of acetic acid. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. distilled white vinegar (figure \(\pageindex{2}\)) is a solution of acetic acid, \(ch_3co_2h\), in water. weak acids and bases may be just as dangerous as their stronger counterparts. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.numerade.com
SOLVEDA typical sample of vinegar has a pH of 3.0. Assuming that vinegar is only an aqueous Vinegar Is An Aqueous Solution Of Acetic Acid For example, vinegar is an aqueous. vinegar is an aqueous solution of acetic acid (ch3cooh). acetic acid is a carboxylic acid with the formula ch3cooh, whereas vinegar is an aqueous solution of acetic acid and trace. vinegar is an aqueous solution of acetic acid. in this experiment, a technique known as a titration will be used. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.alamy.com
Acetic acid molecule. Vinegar is an aqueous solution of acetic acid. Stylized skeletal formula Vinegar Is An Aqueous Solution Of Acetic Acid If the mass percent acetic acid in a bottle of vinegar is 9.0 % and the density of. For example, vinegar is an aqueous. acetic acid is a carboxylic acid with the formula ch3cooh, whereas vinegar is an aqueous solution of acetic acid and trace. in this experiment, a technique known as a titration will be used to. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.alamy.com
Acetic acid molecule. Vinegar is an aqueous solution of acetic acid. Blue skeletal formula on Vinegar Is An Aqueous Solution Of Acetic Acid For example, vinegar is an aqueous. vinegar is an aqueous solution of acetic acid. distilled white vinegar (figure \(\pageindex{2}\)) is a solution of acetic acid, \(ch_3co_2h\), in water. vinegar is an aqueous solution of acetic acid (ch3cooh). in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. Vinegar Is An Aqueous Solution Of Acetic Acid.
From stock.adobe.com
Acetic acid molecule. Vinegar is an aqueous solution of acetic acid. Skeletal formula. Stock Vinegar Is An Aqueous Solution Of Acetic Acid For example, vinegar is an aqueous. distilled white vinegar (figure \(\pageindex{2}\)) is a solution of acetic acid, \(ch_3co_2h\), in water. vinegar is an aqueous solution of acetic acid (ch3cooh). acetic acid is a carboxylic acid with the formula ch3cooh, whereas vinegar is an aqueous solution of acetic acid and trace. in this experiment, a technique known. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.alamy.com
Acetic acid molecule. Vinegar is an aqueous solution of acetic acid. Skeletal formula Stock Vinegar Is An Aqueous Solution Of Acetic Acid If the mass percent acetic acid in a bottle of vinegar is 9.0 % and the density of. For example, vinegar is an aqueous. distilled white vinegar (figure \(\pageindex{2}\)) is a solution of acetic acid, \(ch_3co_2h\), in water. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.dreamstime.com
Acetic Acid Molecule. Vinegar is an Aqueous Solution of Acetic Acid. Skeletal Formula Stock Vinegar Is An Aqueous Solution Of Acetic Acid distilled white vinegar (figure \(\pageindex{2}\)) is a solution of acetic acid, \(ch_3co_2h\), in water. vinegar is an aqueous solution of acetic acid. acetic acid is a carboxylic acid with the formula ch3cooh, whereas vinegar is an aqueous solution of acetic acid and trace. If the mass percent acetic acid in a bottle of vinegar is 9.0 %. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.dreamstime.com
Acetic Acid Molecule. Vinegar is an Aqueous Solution of Acetic Acid. Skeletal Formula Stock Vinegar Is An Aqueous Solution Of Acetic Acid vinegar is an aqueous solution of acetic acid (ch3cooh). vinegar is an aqueous solution of acetic acid. weak acids and bases may be just as dangerous as their stronger counterparts when concentrated. acetic acid is a carboxylic acid with the formula ch3cooh, whereas vinegar is an aqueous solution of acetic acid and trace. distilled white. Vinegar Is An Aqueous Solution Of Acetic Acid.
From www.dreamstime.com
Acetic Acid Molecule. Vinegar is an Aqueous Solution of Acetic Acid. Skeletal Formula Stock Vinegar Is An Aqueous Solution Of Acetic Acid If the mass percent acetic acid in a bottle of vinegar is 9.0 % and the density of. For example, vinegar is an aqueous. weak acids and bases may be just as dangerous as their stronger counterparts when concentrated. vinegar is an aqueous solution of acetic acid (ch3cooh). By law, it must contain 4 g acetic acid (ch3co2h. Vinegar Is An Aqueous Solution Of Acetic Acid.